- SEIKA Digital Image Corporation.
- Measurement
- LIF ( Laser Induced Fluorescence ) | Overview & Principle
LIF ( Laser Induced Fluorescence ) | Overview & Principle
LIF(Laser Induced Fluorescence) is
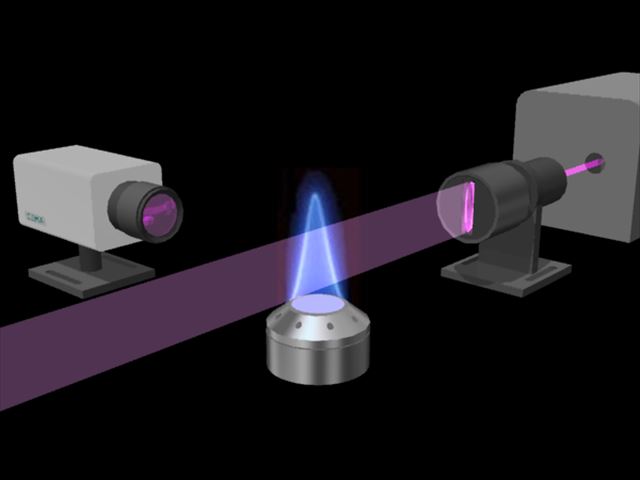
LIF (Laser Induced Fluorescence) is a generic term for technologies to excite specific molecules contained in a measurement target by a single wavelength light source such as laser and observe the fluorescence from the molecule.
It is possible to identify the gas species such as acetone, OH, CH, NO, etc., know the concentrations of atoms / molecules from the intensity of the excitation spectrum, and know the temperature from the spectral distribution.
In addition, it can be applied to various fields such as PLIF which measures multidimensional species, temperature, speed etc. simultaneously, and liquid film thickness measurement.
There are various ways to measure the gas species in the flame. Point measurement, line measurement and area (2D) It will also change depending on measurement and usage.
The table is an example of each measurement method, measurable gas molecular species.
横スクロールでご覧いただけます。
| Measurement method | Measurement system | Gas molecular species |
|---|---|---|
| Points | Self-emission spectrum | OH, CH, C2 |
| Points | CARS | N2, O2 |
| Line | Raman scattering method | OH, NO, NO2, C2, CH...etc |
| Line | TDLAS | CH4, CO, CO2, O2, HCl, NH3, HC...etc |
| Area |
PLIF | OH, C, NO, O2, CO See specification example for each gas |
Here we will inform you about the PLIF method which is one of area measurements.
Feature of Our LIF Sytem Software "Koncerto-LIF"
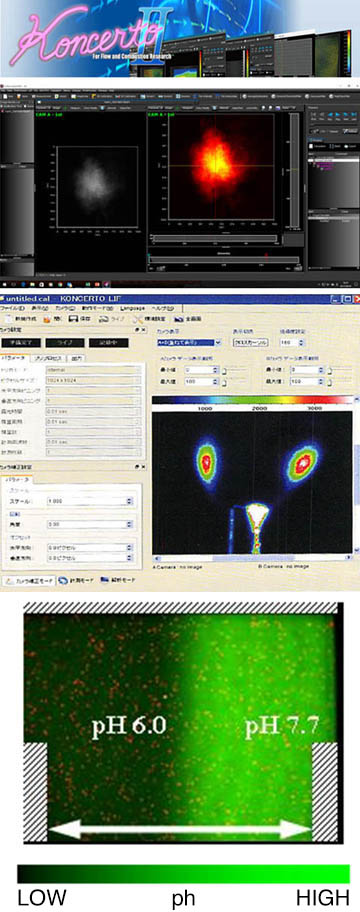
Koncerto LIF ( control and analysis software developed by Saika digital image ) is an integrated imaging measurement software with functions necessary for controlling ultra-sensitive cameras such as ICCD cameras and EMCCD cameras, displaying and analyzing high bit images.
- Camera control function
It corresponds to various manufacturers and models. Simultaneous control of multiple cameras, image display is possible. - 2 Camera alignment function
It is an adjustment function for equalizing the angle of view of the two cameras.Calibration target is used for angle of view adjustment. First of all, use the camera alignment assist function to make the angle of view of the two cameras as identical as possible. Next, fine-tune the error that can not be physically adjusted with software. - Timing controller control
Timing controller LC880 is controlled in real time and synchronous control of the equipment making up the system such as camera and laser is performed. -
Laser intensity monitor
In LIF, luminance values of each pixel are often converted to density, temperature, etc., and variations between laser intensity shots lead to measurement errors. With KoncertoLIF, it is possible to measure and record the laser intensity at the time of image acquisition and correct the measurement error due to fluctuation of laser intensity between frames. - Display of high bit image
It is possible to display high bit images such as 16 bit in an easy-to-understand manner. It has various functions such as XY histogram along the cross cursor, global histogram display function, easy to use display range setting function and so on. - Analysis / display function
Real time camera image display function, pseudo color display, average calculation, arithmetic operation, correction of dispersion of spatial brightness distribution, correction of variations in strength between shots of laser, image correction, calibration (scale factor, scalar quantity) , It has various functions necessary for micro image analysis. - Confocal scanner control
Yokogawa Electric CSU series control is possible, micro imaging high speed, high spatial resolution is possible. - Piezo focus scanner control
It is possible to scan the focal plane at high speed with a piezo focus scanner for micro imaging. Scanning can be performed in conjunction with frames of various cameras, and various program scans such as time lapse shooting and micro PIV shooting are possible.
LIF Variation
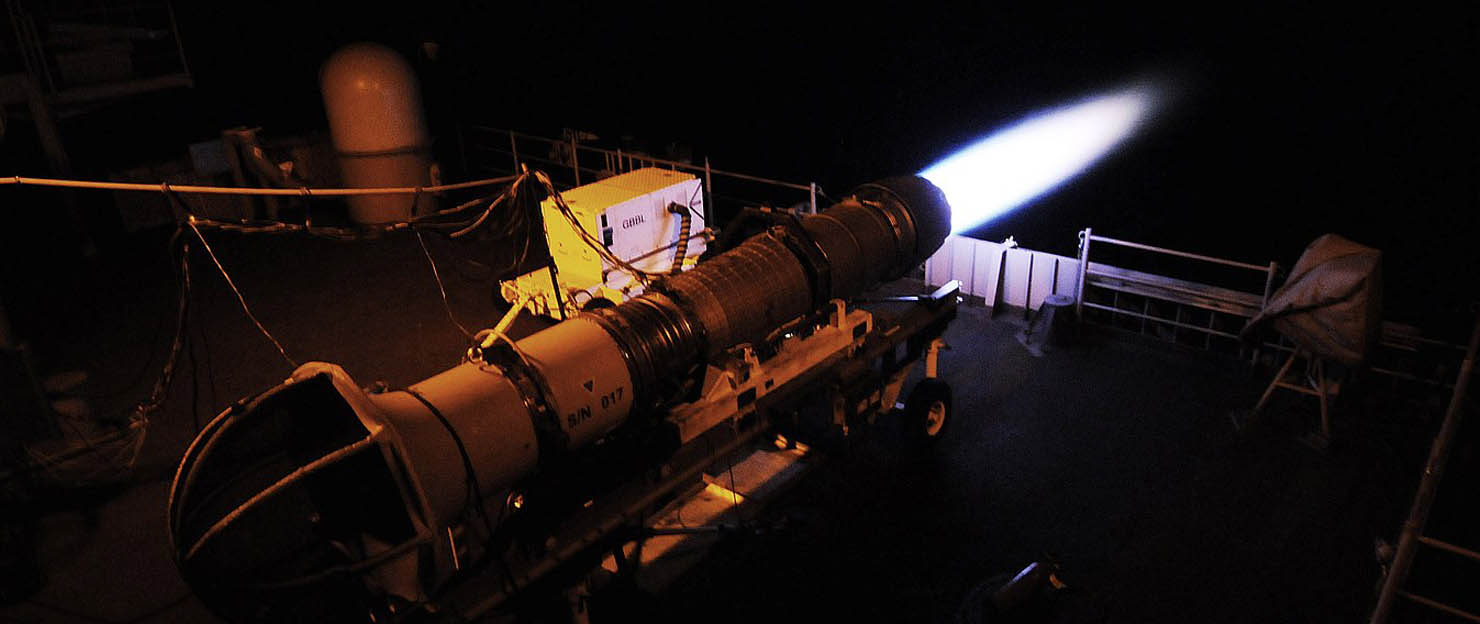
Combustion measurement ( PLIF )
In the combustion measurement by LIF, it is possible to measure the molecular species of gas such as acetone, OH, CH, NO and their instantaneous distribution. Among LIFs, planar LIF measurement by images is called PLIF (Image Laser Induced Fluorescence, Planer Laser Induced Fluorescence). PLIF is an LIF system that uses an ultra-sensitive camera such as ICCD and is a very effective technique for combustion measurement and analysis.
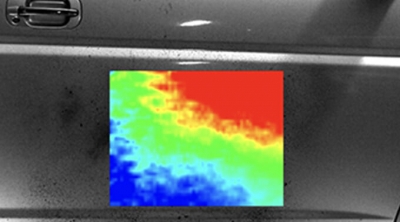
Oil film thickness measurement
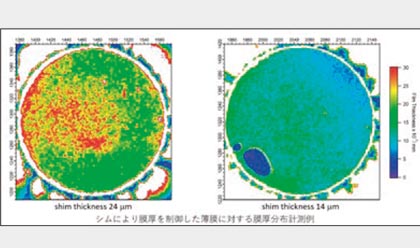
This technology measures the film thickness of oil film such as bearing and oil seal by LIF (laser induced fluorescence method). Because it is measured by image analysis, it is possible to measure samples without external influences. Quantitatively measure film thickness in the range of sub μm to 100 μm. By adding a fluorescent agent to heptane etc., it becomes possible to visualize the state of atomization of the injector and the state of adhesion to the port, and it is possible to evaluate the atomization status of the injector.
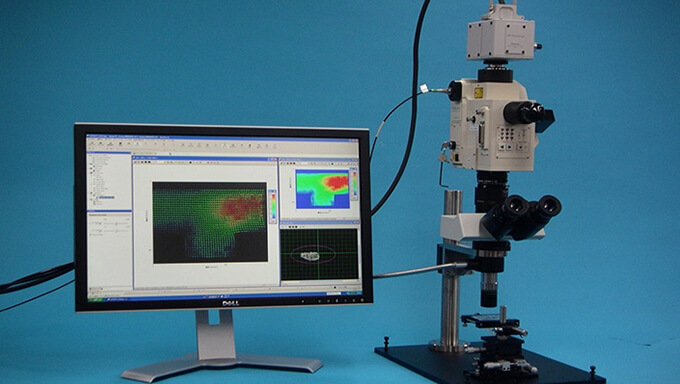
micro LIF
For micro LIF, it is possible to measure concentration distribution, temperature distribution, diffusion, mixing, reaction, PH etc. A dedicated micro optical system can coaxially epi-illuminate a high power UV pulse laser which is impossible with a general microscope.
Multi PLIF
PLIF can perhaps measure child species, temperature, speed etc at the same time. For example, acetone shows absorption peak at 280 nm and fluorescence is seen around 435 nm. OH radicals are induced by 283 nm and instantaneously fluoresce around 315 nm. These simultaneous measurements are possible.
Principle of LIF
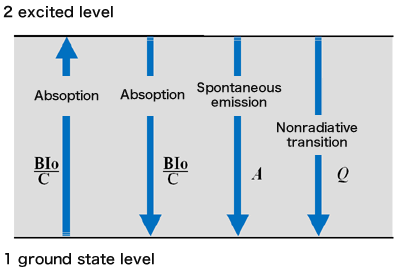
Generally, LIF is a method that excites specific kinds of atoms or molecules and detects the resulting luminescence.The density of the atoms or molecules can be measured from the intensity of the excitation spectrum obtained using this procedure. In addition, the temperature can be measured based on the spectral distribution. LIF has become a major basic spectroscopic technology along with the development of tunable laser technology.
LIF makes it possible to perform high-sensitivity spatial and time-resolved measurements, and it has various practical applications, such as the analysis of reaction mechanisms by detecting intermediate products.
The spectrum that is obtained from observation of the light absorption when the wavelength of an incident laser light is changed is called the absorption spectrum. However, a precise observation of absorption spectra is very difficult, because light changes in the intensity should be detected. On the other hand, the spectrum that is obtained from observation of the fluorescence intensity caused by the radiative transition due to photoexcitation of atoms or molecules is called the fluorescence excitation spectrum.
LIF observes the fluorescence excitation spectrum and obtains the ground state distribution of the atoms or molecules
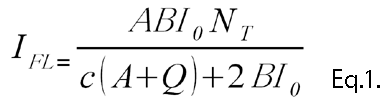
LIF observes the fluorescence emittedspontaneously from excited molecules that were promoted to the excited state through the resonant transition of atoms and molecules.
The chart on the left shows the excitation,emission, and spontaneous emission processes in a simple system consisting of a ground state and one excited state.
Thefluorescence intensity can be described as in Eq .1.
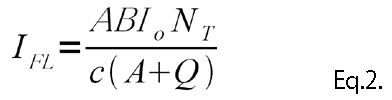
In Eq.1, the meaning of each symbol is as follows:
A, B: Einstein A and B coefficients, respectively
Q: Nonradiative transition rate constant
c: Speed of light
IO: Intensity of the exciting light
NT: Number of atoms or molecules in the ground state prior to excitation
Eq.1 shows that NT can be calculated by observing the IFL. When the intensity of the exciting light is weak, Eq.2 can be adapted, and the ILF is proportional to the intensity of the exciting light.
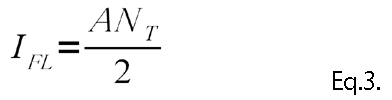
If the exciting light is very intense, the intensity of the fluorescence is independent of IO. IFL in this state is called the saturated fluorescence. Except in special cases, LIF measurements are performed in a state without saturation.
As shown in Eq.1, the intensity of the fluorescence depends on the nonradiative transition rate constant, namely, the quantum efficiency (A/(A+Q)). Therefore, the number of molecules in the ground state can be obtained from the intensity of the fluorescence, and the integration of the observed data at different excitation wavelengths gives the spectrum.
An excitation spectrum is obtained using this procedure. A nonradiative transition is the process of returning to the ground state from an excited state without emitting fluorescence. Various processes are involved in nonradiative transitions, e.g., collisions with coexisting gas atoms or molecules and the level structure of the molecule itself. Consequently, the excitation spectrum and the absorption spectrum don’t always coincide. In particular, OH is a common radical in combustion systems, and many practical examples of its measurements have been reported. However, most of these reports include only relative molecule density measurements. To perform absolute measurements, the specification of the measured area (the sheet light area in PLIF) is necessary.
Applications

- Radical measurement in engine combustion chamber
- Radical measurement of burner
- Radical measurement of plasma
- Temperature measurement
-
Concentration measurement
- PH measurement
- Mixed state measurement
LIF system components
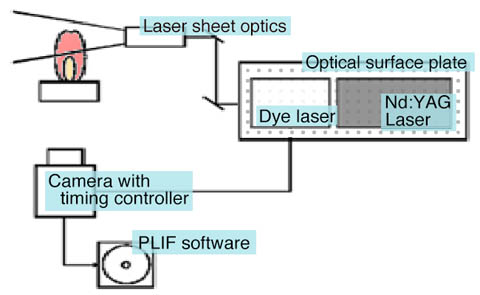
The basic optical system for observing LIF is shown in the figure.The excitation light is made to enter the observation region where the target molecule exists.
Then resonantly excite the target atoms and molecules.
Collect the generated fluorescence with a lens with a relatively large aperture set in a direction perpendicular to the optical axis of the excitation light and detect it with a detector such as a camera.
Dye lasers whose wavelengths can be changed over a wide range and their harmonics are used as excitation light sources.
Gas type lineup
In the combustion measurement by LIF, it is possible to measure the molecular species of gas such as acetone, OH, CH, NO and their instantaneous distribution.
The system configuration varies depending on each gas type.
See specification example for each gas.
Related Products
Contact
Any inquiry or request for information, please click here.